Reimagine
your approachFor adults with advanced RCC
WELIREG is indicated for the treatment of adult patients with advanced renal cell carcinoma (RCC) following a programmed death receptor-1 (PD-1) or programmed death-ligand 1 (PD-L1) inhibitor and a vascular endothelial growth factor tyrosine kinase inhibitor (VEGF-TKI).



your approachFor adults with advanced RCC
WELIREG is indicated for the treatment of adult patients with advanced renal cell carcinoma (RCC) following a programmed death receptor-1 (PD-1) or programmed death-ligand 1 (PD-L1) inhibitor and a vascular endothelial growth factor tyrosine kinase inhibitor (VEGF-TKI).
National Comprehensive Cancer Network® (NCCN®) Recommended Option
Belzutifan (WELIREG) is the only HIF-2α inhibitor included as an other recommended subsequent
therapy option (NCCN Category 2A) for patients with advanced clear cell RCC following prior PD-1 or
PD-L1 inhibitor and VEGF-TKI therapies.1,a
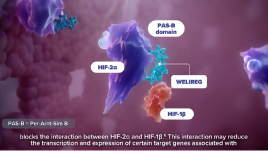
Mechanism of Action
The approval of WELIREG marks the first treatment option in a novel therapeutic class available for your appropriate patients with advanced RCC since 2015.2
Efficacy
See primary analysis data from LITESPARK-005 and the study design that included only patients who received prior treatment with both anti–PD-1/L-1 and VEGF-TKI therapies, in sequence or in combination.
2L+ Treatment Sequencing
WELIREG may be considered as early as the second line for your appropriate patients with advanced RCC following treatment with both anti–PD-1/L1 and VEGF-TKI therapies.
Efficacy Data From
LITESPARK-005
Learn more about efficacy data from the primary analysis of LITESPARK-005. Data from an extended analysis with a median follow-up time of 25.7 months are also available.

Download this flashcard for an overview of the NCCN Guidelines for belzutifan (WELIREG) in adult patients with previously treated 2L+ advanced RCC.
View more resourcesaThis regimen is for patients that have received a PD-1 or PD-L1 inhibitor and a VEGF-TKI.
Category 2A = Based upon lower-level evidence, there is uniform NCCN consensus (≥85% support of the Panel) that the intervention is appropriate.
NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
NCCN = National Comprehensive Cancer Network; 2L+ = second line or later.
References: 1. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Kidney Cancer V.2.2025. © National Comprehensive Cancer Network, Inc. 2024. All rights reserved. Accessed September 9, 2024. To view the most recent and complete version of the guideline, go online to NCCN.org. 2. Yap NY, Khoo WT, Perumal K, et al. Practical updates in medical therapy for advanced and metastatic renal cell carcinoma. Urol Sci. 2018;29(3):120–128.
WELIREG may be considered as early as the second line for your appropriate patients with advanced RCC following treatment with both anti–PD-1/L1 and VEGF-TKI therapies